Solutions for water-sensitive pharmaceutical preparations-pharmaceutical excipients
- Categories:Application Case
- Author:
- Origin:
- Time of issue:2021-04-26 15:47
- Views:
 ;
;
Solutions for water-sensitive pharmaceutical preparations-pharmaceutical excipients
(Summary description)Introduction: The instability of drugs may lead to short shelf life and even product recalls. We have conducted sufficient research on the degradation mechanism of APIs and drugs. Although a mature science has not yet been formed, a reasonable understanding has been obtained. The two most common degradation methods for APIs and drugs are hydrolysis and oxidation. Water is ubiquitous in the environment, and the related hydrolysis reaction is the most common of the two main degradation mechanisms. In the framework of "Quality by Design (QbD)", the basis for the realization of control strategies is an in-depth understanding of the critical quality attributes (CQA) of the product.
- Categories:Application Case
- Author:
- Origin:
- Time of issue:2021-04-26 15:47
- Views:
Introduction:
The instability of drugs may lead to short shelf life and even product recalls. We have conducted sufficient research on the degradation mechanism of APIs and drugs. Although a mature science has not yet been formed, a reasonable understanding has been obtained. The two most common degradation methods for APIs and drugs are hydrolysis and oxidation. Water is ubiquitous in the environment, and the related hydrolysis reaction is the most common of the two main degradation mechanisms. In the framework of "Quality by Design (QbD)", the basis for the realization of control strategies is an in-depth understanding of the critical quality attributes (CQA) of the product. Control accessories (CQA) are part of our control strategy.
Accessories control
Accessories
Both excipients and API contain water, which can be in a bound or unbound state. Common excipients are divided into 4 categories: (I) non-hygroscopic, (II) slightly hygroscopic, (III) moderately hygroscopic or (IV) very hygroscopic. Water activity is a quantitative indicator of free-flowing water available in the system, and this value is equivalent to the relative humidity in a closed environment. Then, the inherent chemical stability of the system can be correlated with the water activity
Excipient compatibility testing can usually identify excipients that adversely affect hydrolytic instability, but the choice of excipients needs to balance the best stability, best manufacturability, and best biopharmaceutical properties . Therefore, it may be unrealistic to completely avoid auxiliary materials or production processes that may promote hydrolysis.
Hygroscopic component
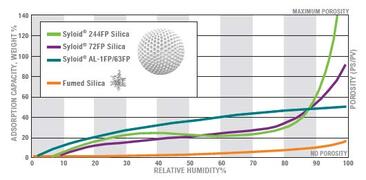
Certain excipients can strongly absorb "free" water, thereby removing free water in the formulation and reducing water activity, and ultimately stabilize the water-sensitive API. Such as gel silica (Syloid®FP series).
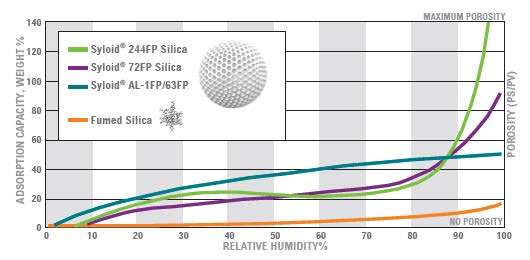
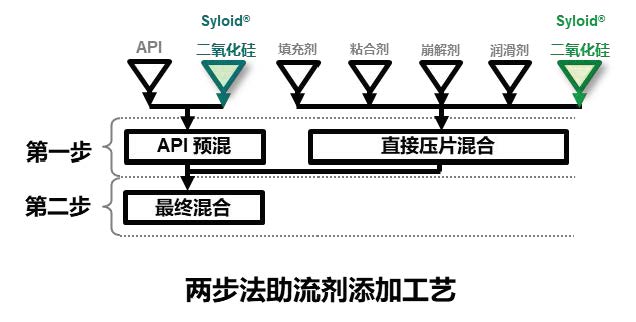
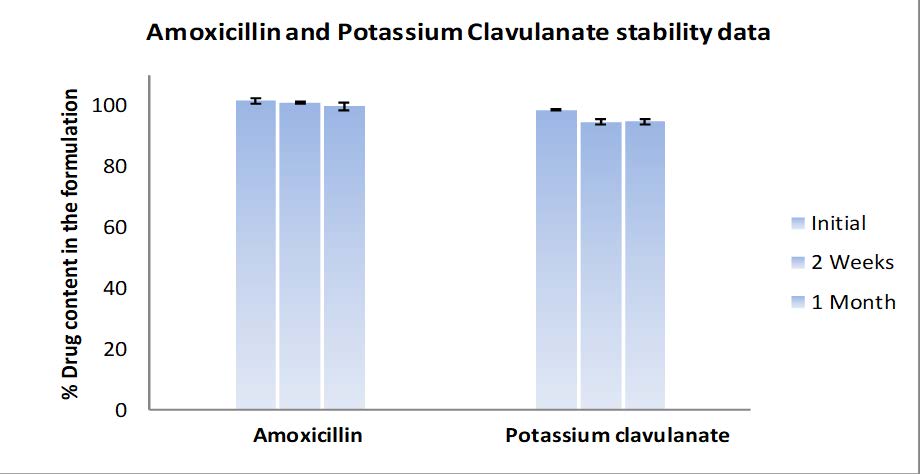
Stable experimental results
Excipients from different sources
If there is only a single source of key excipients, it may pose a major business challenge (business continuity issues); however, it is well known that the use of excipients from different suppliers may increase the variability of the performance of the excipients. For certain excipients, the instability of the drug is usually related to its residues. Therefore, the impurity spectrum of excipients is different, which may cause different stability problems of the drug. However, instability caused by impurities is not the only way for excipients from different sources to affect the physical and chemical stability of the drug. Especially in terms of physical properties, the auxiliary material parameters that characterize the particle size or bulk properties may be different.
Excipients with low water content
The auxiliary materials can be dried before use, but this usually affects their compressibility. Or, you can choose different models of the same auxiliary materials, that is, low-moisture models, which may be used to replace the higher-moisture auxiliary materials currently in use. Obviously, the latter method has some impact on costs, but these may enhance stability and extend shelf life. For oral solid dosage forms, this strategy should be aimed at diluents (fillers), because these excipients account for the largest weight percentage of tablets. The diluent contributes to the fluidity and compressibility of the material.
We compared the moisture content, particle size and bulk density of the most common fillers, different grades of Comprecel® MCC. The data is summarized in the table below.
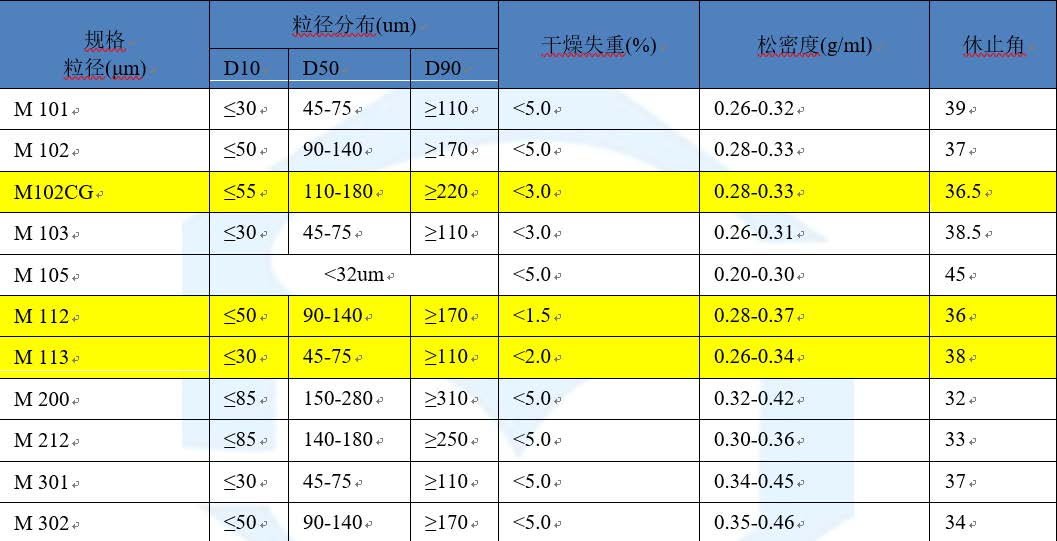

in conclusion
In short, pharmaceutical excipients can play a stabilizing or destabilizing effect, which mainly depends on the API. Knowing this point is critical to the development of drugs. For APIs that are easily hydrolyzed, it is also important to understand the effects of excipients. Therefore, it is recommended to choose excipients and their grades wisely through excipient compatibility experiments. There are many overlaps between the control of prescription and the control of excipients. Finally, the impact of process and packaging on physical and/or chemical stability should not be underestimated.
Copyright © Shanghai Shenmei Pharmaceutical Development Technology Co., Ltd. All Rights Reserved 沪ICP备17050915号-4 POWERED BY 300.CN






